From the journals: August 2019
How an oyster protein drives shell formation
Matrix proteins rich in acidic asparagine and glutamine residues are needed to form biominerals such as bones and shells, with the carboxylates of these residues serving as possible calcium-binding groups. Dong Yang and a team at Tsinghua University have characterized the pearl oyster protein N25, which contains high proportions of proline, serine and basic lysine residues, finding that it also affects biomineral formation, attaching to crystal surfaces and delaying a morphological transition. , published in the Journal of Biological Chemistry, shed light on biomineral formation mechanisms and raise intriguing questions about potential synergisms among distinct matrix proteins.
A better way to count eicosanoids
Eicosanoids are signaling lipids that play a role in pain and inflammation. They function in diseases such as asthma and cancer, making them clinically relevant biomarkers. In published in the Journal of Lipid Research, Cristina G贸mez and an international team present an improved method of extracting and quantifying eicosanoids from human urine, suitable for use with groups in large and diverse clinical trials. The included metabolites cover major synthetic pathways, including prostaglandins, leukotrienes and isoprostanes.
The researchers were able to extract 32 eicosanoids rapidly, reliably and precisely from urine, with average extraction recoveries of over 90%, then quantify them by liquid chromatography and mass spectroscopy. Their method includes a faster and more streamlined protocol that is suitable for large-scale studies and small sample volumes (1.4 mL of urine for the complete workflow). This method could be used for numerous types of studies of various populations, including children.
Making neurotoxic plaques miss their mark
A hallmark of Alzheimer’s disease is the aggregation of beta-amyloid plaques in the brain that exhibit neurotoxic properties and contribute to neurodegeneration. In the Journal of Biological Chemistry, British that activating an endogenous enzyme that chops off beta-amyloid targets from the surface of neurons curbs the neurotoxic effects of the plaques.
Oligomers of beta-amyloid, or ABOs, are particularly toxic and bind to receptors on the neuronal surface, triggering signaling pathways and the generation of reactive oxygen species, which impair cell function and survival. Recent research has shown that one of these receptors, cellular prion protein, or PrPC, can be removed from the cell surface by the enzyme ADAM10. and colleagues at the University of Manchester and the University of Oxford sought to increase the activity of this enzyme and, in turn, reduce the toxic activity of ABOs.
Previous work has shown that the muscarinic agonist carbachol and the vitamin A analog aciretin can activate ADAM10. Jarosz–Griffiths’ team exposed human cell types to these compounds and found that either could promote shedding of PrPC from the surface of neuroblastoma cells or induced pluripotent stem cell-derived neurons. These molecules blocked the binding of ABO and reduced its toxic effects. Knockdown of ADAM10 diminished these benefits, indicating that activation of this specific enzyme is critical for PrPC shedding and protection from ABOs.
These results demonstrate that reducing neuronal targets for ABOs by activating ADAM10 could have significant therapeutic potential for Alzheimer’s disease patients.
— Jonathan Griffin
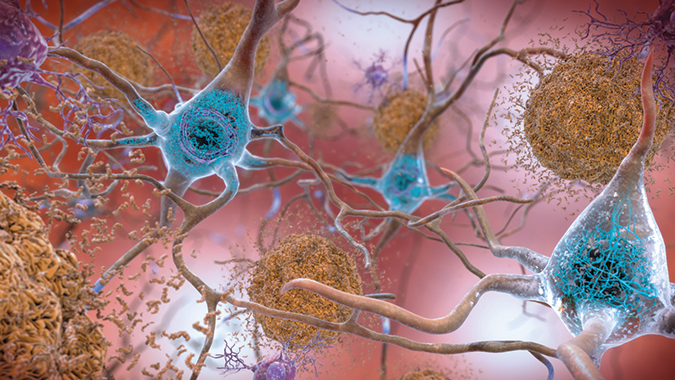 In the Alzheimer’s-affected brain, abnormal levels of beta-amyloid protein clump together to form plaques, shown in brown, that accumulate between neurons, disrupting cell function and survival.National Institute on Aging, NIH
In the Alzheimer’s-affected brain, abnormal levels of beta-amyloid protein clump together to form plaques, shown in brown, that accumulate between neurons, disrupting cell function and survival.National Institute on Aging, NIH
Crystal structures could improve pain meds
COX-2 is a target of the nonsteroidal anti-inflammatory drugs, or NSAIDs, used to manage pain and fever. COX inhibitors containing an indomethacin scaffold are selective for the inflammation-induced COX-2 over the constitutively expressed COX-1, but the basis for this selectivity has not been established definitively. Shu Xu and a team of U.S. researchers have in the Journal of Biological Chemistry the first crystal structures of COX-2 in which the intact derivatives of the NSAID indomethacin are fully visible, confirming that compound binding extends to a unique lobby of the protein and providing critical details to inform future inhibitor design.
How a tree responds to infection
Fast-growing trees of the Paulownia genus have been cultivated in China for centuries. They commonly are found in parks and gardens, and the wood is used to make furniture and musical instruments. Phytoplasma bacteria pose a major threat to these trees by causing Paulownia witches’ broom, or PaWB, a disease characterized by stunted growth and other malformations. Phytoplasmas infect more than 1,000 plant species, resulting in significant economic losses in agriculture and horticulture.
Post-translational modifications such as lysine acetylation and succinylation are known to be involved in plant response to pathogens. However, the functions of these modifications during phytoplasma infection had not been explored previously. In published in the journal Molecular & Cellular Proteomics, Yabing Cao and colleagues from the Henan Agricultural University in China write that acetylation may be more important than succinylation in response to phytoplasma infection in Paulownia tomentosa seedlings. They showed that rubisco and protochlorophyllide reductase, enzymes needed for chlorophyll and starch synthesis, are acetylated at specific sites in phytoplasma-infected seedlings



